|
|||||||
|
Systematic Study of Amyloid Beta Peptides: Implications for Alzheimer’s Disease |
|||||||
|
The amyloid beta peptide particularly the 40 and 42 amino acid residues are the responsible for plaque formation in Alzheimer's disease (AD) patients. Extra cellular plaque formation has been recognized after incessant investigations along with the formation of intracellular tau protein tangles as the hallmarks of AD. Our investigation is focused on studying the formation of AD plaques. Under normal conditions there is an anabolic/catabolic equilibrium of the Aß peptide; therefore, it is believed that the formation of the plaque does not take place. On the other hand, the neurons' surface may play an important role in the adhesion mechanisms of the Aß peptide. Our experiments show that the neuron surfaces along with the media conditions may be the most important causes for progressive formation of plaques. We have incubated rigid supports (mica) and soft biomimetic substrates (lipid bilayers on top of a PEG cushion layer drafted onto a silica surface) with the three different conformations of the Aß peptide (monomeric, oligomeric and fibrils structures) to determine the adhesion mechanisms associated with in situ plaque formation. |
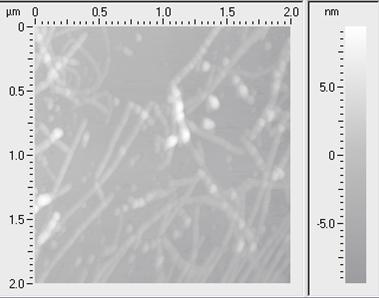
AFM scan of amyloid beta (Ab42) fibrils after 92 h of incubation. The fibrils deposited on a rigid substrate.
|
||||||
|
The characterization of such biomimetic membranes has been studied by using Atomic Force Microscopy (AFM) in liquid environments. Our results show that these lipid bilayers are highly mobile. Additionally the structure and topography characteristics of the Aß conformations have been followed with atomic force microscopy (AFM). The kinetics and rates of adhesion have been measured with attenuated total reflection Fourier transform infrared (ATR-FTIR spectroscopy. Our results show the progress of the plaques' formation with time where simple monomers deposit on the substrates and allow the development of oligomeric species. The oligomers then grow into fibril-like structures leading finally to the plaques that eventually are seen to insulate real neurons and stop them from the synapse process. The ultimate outcome of this investigation will contribute to understand, prevent and determine possible mechanisms for removing AD plaques. |
|||||||